Table of Contents
- Atmospheric pressure
- Mercury barometer for measuring atmospheric pressure
- Measurement of pressure of a gas using open tube manometer
- Absolute pressure and gauge pressure
Atmospheric pressure
The gaseous envelope surrounding the earth is called the atmosphere. The pressure exerted by the atmosphere is called atmospheric pressure.
The force exerted by air column of air on a unit area of the earth’s surface is equal to the atmospheric pressure. The atmospheric pressure at sea level is 1.013 × 105 Nm-2 or Pa.
Mercury barometer for measuring atmospheric pressure
Torricelli’s experiment of measuring atmospheric pressure.
Mercury barometer
An Italian scientist E. Torricelli was first to device a method for measuring atmospheric pressure accurately. It is called a simple barometer. Aim long glass tube closed at one end is filled with clean and dry mercury. After closing the end of the tube with the thumb, the tube is inverted into a dish of mercury. As the thumb is removed, the mercury level in the tube falls down a little and comes to rest at a vertical height of 76 cm above the mercury level in the dish.
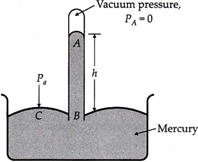
The space above mercury in the tube is almost a perfect vacuum and is called Torricellian vacuum. Therefore, pressure PA =0. Consider a point C on the mercury surface in the dish and point B in the tube at the same horizontal level. Then
PB = PC = Atmospheric pressure, Pa
If h is the height of mercury column and ρ is. the density of mercury, then
PB = PA+hρg
or Pa = 0 + h ρ g
or Pa = h ρ g
For a mercury barometer, h =76 cm =0.76 m, ρ =13.6 × 103kg m-3, g = 9.8 ms-2, therefore
Atmospheric pressure,
Pa =0.76 × 13.6 × 103 × 9.8 =1.013 × 105 Pa.
Measurement of pressure of a gas using open tube manometer
Open-tube manometer. It is a simple device used to measure the pressure of a gas enclosed in a vessel. It consists of a Lf-tube containing some liquid. One end of the tube is open to the atmosphere and the other end is connected to the vessel.
The total pressure P of the gas is equal to the pressure at A Thus
P = PA = PC + h ρ g or P = Pa + h ρ g
where Pa is the atmospheric pressure, h = BC = difference in the levels of the liquid in the two arms and ρ is the density of the liquid.

Absolute pressure and gauge pressure
Let’s discuss the differences between absolute and gauge pressure.
The total or actual pressure P at a point is called absolute pressure. Gauge pressure is the difference between the actual pressure (or absolute pressure) at a point and the atmospheric pressure, i.e., Pg = P – Pa = h ρ g
The gauge pressure is proportional to h. Many pressure measuring devices directly measure the gauge pressure. These include the tyre pressure gauge and the blood pressure gauge (sphygmomanometer).
